The author is a senior extension associate with Penn State University’s Department of Animal Science.
Hydrogen sulfide (H2S) is undoubtedly the most dangerous gas associated with manure storage and handling. The United States Occupational Safety and Health Administration (OSHA) lists acceptable exposure concentration limits for H2S as 20 parts per million (ppm) with a 10-minute exposure maximum peak over an 8-hour shift as 50 ppm. OSHA lists concentrations as low as 700 ppm as the level that can cause immediate collapse and death within minutes, and 1,000 ppm is the level that can cause nearly immediate death.
An unfortunate by-product
Hydrogen sulfide can be produced as a microbial by-product of manure degradation, with production of the deadly gas exacerbated when anaerobic manure storage conditions favor proliferation of sulfate-reducing bacteria. Higher levels of H2S can be produced in manure when sulfur inputs into the manure are elevated from sources such as gypsum bedding, by-product feed ingredients, or sulfur-containing water sources. Temperature also impacts H2S production, since microbial activity increases in warmer manure. Higher H2S concentration will lead to greater emissions.
Emissions can occur one molecule at a time when H2S moves across a gradient from concentrated solutions at the manure surface to low concentrations in the air above the manure. However, since H2S is only slightly soluble in water, the molecule “wants” to come out of solution as concentrations grow.
In deeper anaerobic areas of a manure storage, where microbial production continually supplies H2S, concentrations of gas can elevate beyond their solubility limit and escape the solution by coming together to form bubbles and release from manure through ebullition processes.
Other gases produced from microbial degradation of manure, such as methane and carbon dioxide, also form bubbles. Bubbles can contain a mixture of different gases.
Disturbing manure with processes such as agitation are expected to intensify H2S release. Violent manure movement brings higher concentrations found in the depth of manure to the surface, and shifting concentration gradients favor release of the poorly soluble molecule.
Worried about the weight
A characteristic that makes H2S dangerous is that it has a molecular weight of 34 grams per mole (g/mol), compared to the weight of dry air at 29 g/mol. The weight of air decreases with moisture, since water weighs just 18 g/mol. This means that certain atmospheric conditions and locations can raise the risk of H2S accumulation close to the ground, in low areas, or in confined spaces.
If air is still, the gas that leaves the manure surface may not be carried away, and since H2S is heavy, continual release can accumulate gas just above the manure surface. Such pooling of H2S can lead to deadly concentrations.
A report from the Centers for Disease Control and Prevention highlights how all these factors led to the 2016 death of a worker and 13 cattle at a Wisconsin farm. The farm’s ration included a distillers syrup that contained sulfur above dietary recommendations, and the manure storage was deep and anaerobic. The incident occurred in mid-August when summer temperatures promoted microbial proliferation. Atmospheric circumstances enhanced the pooling characteristics of H2S because there was no wind. Humidity was high, thermal inversion conditions existed, and the worker had performed manure agitation.
In Pennsylvania, studies were conducted that compared H2S emissions during agitation at dairy farms that either used or did not use gypsum bedding and provided practical recommendations concerning manure handling. Gypsum bedding (CaSO4), derived from recycled drywall material from the construction industry, was shown to elevate H2S emissions during manure storage agitation.
On one dairy that used gypsum bedding, the ability of heavy H2S gas to pool provided a great learning opportunity. At this farm, measurements were made around the perimeter of a circular concrete manure storage (see photo). During agitation, when wind moved from the west, the maximum downwind H2S monitor reading was 64 ppm, a level above OSHA standards but not deadly. When the wind shifted and moved from the southeast, the downwind H2S monitor measured 500 ppm, the maximum reading the monitor could register.
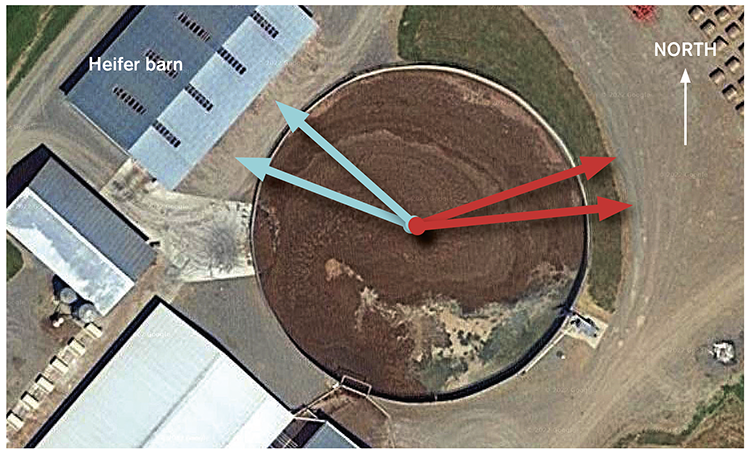
What was the difference between these positions during this agitation event? We believe that as the breeze moved over the heifer barn seen at the top left of the aerial photo, a dead space was created, much like an eddy in a stream, that allowed the heavy gas to settle, pool, and become concentrated.
Areas such as this may not appear dangerous, but people should avoid working next to structures, like this heifer barn, that can provide obstruction to free air movement. When possible, manure handlers should position themselves so that wind travels across their position before crossing the manure storage. This can be phrased as “keeping the wind at your back.”
Avoid these spaces
Low-lying areas and confined spaces where H2S may pool should always be avoided. There are three characteristics OSHA uses to define areas that are confined spaces:
1. Large enough to enter
2. Limited means of entry and exit
3. Not designed for continuous worker occupancy
These traits pertain to nearly every manure storage since none are designed for continuous worker occupancy. This includes any uncovered, open-air, outdoor storage. For this reason, consider any space inside of a manure storage fence as a confined space and never enter such spaces without proper entry equipment, ventilation, and precautions. Always pull equipment outside of the manure storage area for maintenance.
Hydrogen sulfide is a deadly gas. Agricultural professionals, including manure system designers, nutritionists, farmers, manure handlers, agency personnel, and university employees, should work together to ensure worker and livestock safety.
This article appeared in the May 2022 issue of Journal of Nutrient Management on pages 16 & 17. Not a subscriber? Click to get the print magazine.



